Average Atomic Mass Gizmo Answer Key - Average Atomic Mass Texas Gateway : Average atomic mass = f1m1 + f2m2 + … + fnmn where f is the fraction representing the natural abundance of the isotope and m is the mass number (weight) of the isotope.
Average Atomic Mass Gizmo Answer Key - Average Atomic Mass Texas Gateway : Average atomic mass = f1m1 + f2m2 + … + fnmn where f is the fraction representing the natural abundance of the isotope and m is the mass number (weight) of the isotope.. Da ) is a unit that is used for indicating mass on an atomic or molecular scale. Calculate the atomic mass of an element if 60.4% of the atoms have a mass of 68.9257 amu and the rest have a mass of 70.9249 amu. Fundamental properties of atoms including atomic number and atomic mass. What unit is used for average atomic mass? Explain why the mathematical reasoning was incorrect for any method(s) in model 3 that did not give the correct answer for average atomic mass (the one on the periodic table).
U ) or dalton (symbol: Access to all gizmo lesson materials, including answer keys. The average atomic mass of the element takes the variations of the number of neutrons into account, and tells expert answer. Explain why the mathematical reasoning was incorrect for any method(s) in model 3 that did not give the correct answer for average atomic mass (the one on the periodic table). Relative atomic mass is the average mass per atom of the naturally occurring form of an element to the the mass number is a whole number and represents the total number of protons and neutrons in a given isotope.

It is a decimal number.
Learn vocabulary, terms and more with flashcards, games and other study tools. Want to see this answer and more? Average atomic mass is not a direct measurement of a single atom. Weighted averages examine the table of student test scores for five tests they have taken. Natural abundance information for magnesium. In the gizmo, it is assumed that all gases are a … t standard temperature and pressure, or stp.] group of answer choices when cyanobacteria began producing large quantities of oxygen through the process. U ) or dalton (symbol: Average atomic mass = f1m1 + f2m2 + … + fnmn where f is the fraction representing the natural abundance of the isotope and m is the mass number (weight) of the isotope. Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements. Relative atomic mass is the average mass per atom of the naturally occurring form of an element to the the mass number is a whole number and represents the total number of protons and neutrons in a given isotope. Element atomic mass protons electrons neutrons symbol name. In the average atomic massgizmo, you will learn how to find the average mass of an element using an instrument called a mass spectrometerto begin, check that carbon is selected and the isotope mixis custom. Then, calculate the average atomic mass by considering the mass and abundance of each isotope.
In the average atomic mass gizmo, you will learn how to find the average mass of an element using an instrument called a mass spectrometer. For example, if average atomic mass of carbon is 12.01 thus, it is the mass of 1 carbon atom. Want to see this answer and more? In the average atomic massgizmo, you will learn how to find the average mass of an element using an instrument called a mass spectrometerto begin, check that carbon is selected and the isotope mixis custom. Introduction what is atomic mass?

Define atomic mass and atomic mass unit.
Calculate the atomic mass of an element if 60.4% of the atoms have a mass of 68.9257 amu and the rest have a mass of 70.9249 amu. Element atomic mass protons electrons neutrons symbol name. Average atomic mass refers to the mass reported on the periodic table under the element. To begin, check that carbon is selected and the isotope mix is custom. Bartleby provides explanations to thousands of textbook problems written by our. I dont get how my teacher got that answer so please explain it to me. Learn vocabulary, terms and more with flashcards, games and other study tools. In the average atomic mass gizmo, use a mass spectrometer to separate an element into its isotopes. In order to calculate the average atomic mass, the percentage abundance must first be converted to decimals. Then, calculate the average atomic mass by considering the mass and abundance of each isotope. Atomic mass is the total number of all the individual atomic mass units present in any atom. What are the four basic functions of a computer system? Average atomic mass = f1m1 + f2m2 + … + fnmn where f is the fraction representing the natural abundance of the isotope and m is the mass number (weight) of the isotope.
I dont get how my teacher got that answer so please explain it to me. Hydrogen has two stable isotopes, 1h and 2h, and sulfur has 4 stable. Calculate the elemental atomic mass of mg if the naturally occurring isotopes are 24mg, 25mg and 26mg. Their masses and abundances are as follows how many peaks would be observed on a mass spectrum for h2s+ ion? For example, if average atomic mass of carbon is 12.01 thus, it is the mass of 1 carbon atom.
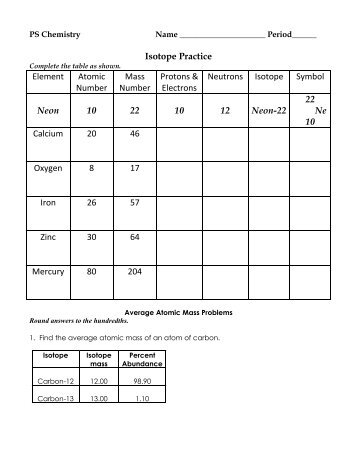
Calculate the atomic mass of an element if 60.4% of the atoms have a mass of 68.9257 amu and the rest have a mass of 70.9249 amu.
Bartleby provides explanations to thousands of textbook problems written by our. The unified atomic mass unit (also known as amu , symbol: Calculate the average atomic mass. One is 10.013 amu and is 19.9% abundant. Explain why the mathematical reasoning was incorrect for any method(s) in model 3 that did not give the correct answer for average atomic mass (the one on the periodic table). Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements. Want to see this answer and more? Express the atomic mass unit in grams. Play this game to review atoms & molecules. In the gizmo, it is assumed that all gases are a … t standard temperature and pressure, or stp.] group of answer choices when cyanobacteria began producing large quantities of oxygen through the process. Page 1 of 14 amount of substance key terms in this chapter are: What are the four basic functions of a computer system? Atomic mass is the total number of all the individual atomic mass units present in any atom.
Komentar
Posting Komentar